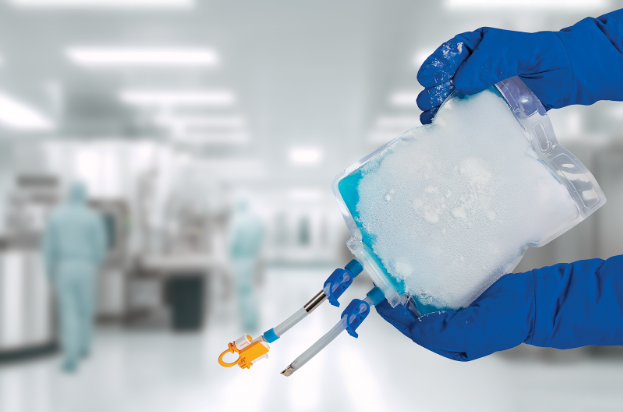
Meissner has broadened the capabilities of its TepoFlex® biocontainer platform by introducing solutions designed specifically for freeze and thaw operations, extending a trusted single-use technology into one of the most challenging stages of biopharmaceutical processing.
The durability and performance of the TepoFlex® polyethylene film platform have been demonstrated over nearly 20 years of industry use. Leveraging these proven characteristics, the biocontainers are designed to withstand the mechanical and thermal stresses associated with repeated freeze and thaw cycles, supporting reliable storage, transport and process integration.
The 2D TepoFlex® biocontainers are suitable for applications requiring freezing at temperatures as low as -80°C. Offered as single-use containers in volumes from 50 mL through to 10 L, they provide flexibility across a broad range of development and manufacturing scenarios.
Engineered for ease of adoption, the flexible biocontainers integrate seamlessly with existing freeze and thaw infrastructure. Compatibility with conventional blast freezers and vertical plate freezers allows manufacturers to implement the solution without major capital investment or workflow disruption.
The expanded TepoFlex® offering provides biopharmaceutical manufacturers with a robust single-use option for safeguarding sensitive products during freeze and thaw processing, while maintaining operational efficiency and flexibility.
Manufacturing is carried out in ISO-certified cleanrooms using qualified materials to ensure biocompatibility and regulatory alignment. In addition, Meissner’s Applications Engineering Team supports customers with consultation and system optimisation, helping tailor assemblies to meet precise application requirements.
Backed by four decades of technical expertise, Meissner continues to prioritise customer experience by partnering closely with clients to understand their objectives and support long-term success.
To discover why leading biopharmaceutical manufacturers trust TepoFlex® for their most essential freeze and thaw applications, visit Meissner’s website at https://www.meissner.com/tepoflex-freeze-thaw where technical resources are available for download.
Additionally, visiting https://www.meissner.com/applications/freeze-thaw/ provides an overview of all of Meissner’s single-use freeze and thaw platform offerings.
About Meissner
Meissner manufactures advanced microfiltration products and therapeutic manufacturing systems for critical pharmaceutical and biopharmaceutical applications, such as sterilization of injectable drugs, and the development and manufacture of life-enhancing/life-saving drugs, therapeutics, biologics, and cell and gene therapies.
Meissner’s manufacturing campuses include a headquarters location in Camarillo, California, USA, a European manufacturing location in Castlebar, Ireland, and buildout of a $250 million facility in Athens, Georgia.
Meissner’s product portfolios are built upon a solid foundation of quality, operational excellence, and technical expertise for delivery of high-performance products that support clients’ critical applications. For more information about Meissner, please visit https://www.meissner.com.